
PathVysion
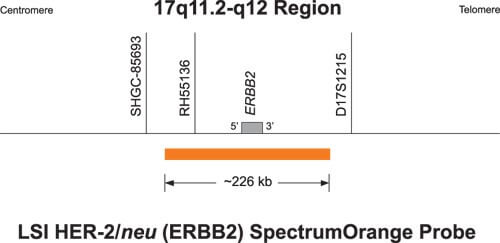
The PathVysion HER-2 DNA Probe Kit
- FDA approved
- Designed to detect amplification of the HER-2/neu gene
- Utilizes patented fluorescence in situ hybridization (FISH) technology
- Applied to formalin-fixed paraffin-embedded (FFPE) human breast cancer tissue specimens
- Intended for use as an adjunct to existing clinical and pathologic information currently used as prognostic factors in stage II, node-positive breast cancer patients
- Indicated as an aid to predict disease-free and overall survival in patients with stage II, node positive breast cancer treated with adjuvant cyclophosphamide, doxorubicin, and 5-fluorouracil (CAF) chemotherapy
- Indicated as an aid in the assessment of patients for whom HERCEPTIN (Trastuzumab) treatment is being considered (see HERCEPTIN package insert)